INTRODUCTION
Alloimmune platelet refractoriness (APR), an insufficient response to platelet transfusion due to anti-HLA alloantibodies, affects ~25% of acute myeloid leukemia (AML) patients. In APR, HLA- or crossmatch-compatible platelet units may not be identifiable, precluding transfusion and increasing hemorrhage risk. Single antigen bead testing (SAB) semi-quantitates antibody levels against cognate HLA antigens in units of mean fluorescence intensity (MFI). MFI >3,000 suggests an incompatible platelet antigen for transfusion. Imlifidase, an IgG-specific cysteine protease, cleaves IgG first into single-cleaved IgG (scIgG) with limited Fc-mediated function, then into inactive F(ab') fragments 1. We previously reported successful desensitization and provision of permissive platelet transfusions in an APR patient following desensitization treatment with imlifidase. Here we report pre- and post-desensitization alloantibody (PDAb) levels in correlation with IgG degradation products and response to single donor platelet transfusion.
CASE
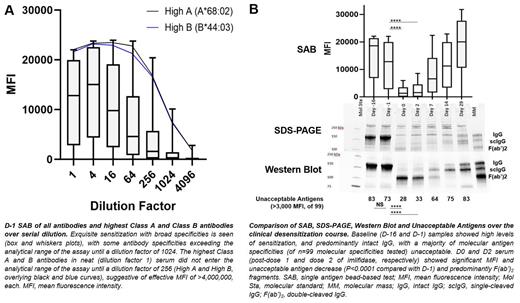
As described previously 2, a 43-year-old highly sensitized (calculated PRA [cPRA] 99.9%) woman with AML and history of life-threatening thrombocytopenic hemorrhage secondary to APR presented for matched-unrelated donor stem cell transplantation. Nationwide, few compatible platelet units were identified to support her through the anticipated platelet nadir. Following conventional desensitization, cPRA did not change significantly. Following imlifidase-desensitization [0.25 mg/kg IV (D-1 and D1)], cPRA decreased significantly. 7 single-donor, antigen-avoidance platelet units produced corrected count increments (CCI) [median (interquartile range) of 6,000 (500-7500)]. The patient experienced no hemorrhage and was discharged stable, post-platelet engraftment on D+19.
METHODS
With IRB approval, patient data and samples (D-16; D-1 post-conventional desensitization with rituximab and therapeutic plasma exchange; D0 post-imlifidase dose 1; D2 post-imlifidase dose 2; and D7, D14 and D29 follow-up samples) were collected. Serial dilutions of patient D-1 serum were tested with SAB (LABScreen single antigen, One Lambda/ThermoFisher, West Hills, California). SDS-PAGE (gradient gel, non-reduced conditions), Western Blot (antiF(ab') detection), and SAB were performed and acceptable antigens (to molecular specificity, targeted by MFI ≤3,000) were compared over the clinical course. Summative MFIs targeting transfused single donor platelet units were compared with 1-hour CCI. Wilcoxon matched-pairs signed rank for matched, nonparametric values and Chi-squared or Fisher's exact for categorical variables were used. P <0.05 was significant.
RESULTS/DISCUSSION
The D-1 sample (post-conventional desensitization, pre-imlifidase) showed exquisite HLA sensitization. The highest Class A and B antibodies demonstrated an effective MFI of >4,000,000, each (Fig. A). PDAb and the number of unacceptable antigens decreased significantly in D0 (post-imlifidase) vs. D-1 (pre-imlifidase) samples; Intact IgG and scIgG were eliminated from the D0 and D2 samples (Fig. B). Of 7 single donor platelet transfusions, the 5 producing adequate response were each targeted by a summative MFI ≤4,000 (median 4,000 MFI, range 300-4,000). The 2 units producing inadequate CCI response were targeted by summative MFI >4,000 (5,050, 4,900-5,200), suggesting a threshold summative MFI precluding adequate response.
CONCLUSIONS
Here we show that the patient's pre-desensitization alloantibody levels significantly exceeded the analytical range of the SAB; PDAb levels declined significantly from baseline, correlating with lack of intact IgG and scIgG following imlifidase; and permissive transfusions (CCI >5,000) were seen to platelets targeted by ≤4,000 summative MFI. These results suggest imlifidase is capable of effective desensitization despite profound baseline sensitization, with rapid cleavage of intact IgG to F(ab'), resulting in the temporary reduction of unacceptable antigens. Larger scale investigation of imlifidase in desensitization of APR patients requiring significant transfusion over a limited period of time, along with investigation into transfusion strategies in desensitized APR, are warranted.
REFERENCES
Am J Transpl. 2022;22(3):691-697.
Blood. 2022;140 (Suppl. 1):8562-3.
.
OffLabel Disclosure:
Järnum:Hansa Biopharma AB: Current Employment, Current equity holder in publicly-traded company. Sundberg:Hansa Biopharma AB: Current Employment.
Imlifidase is an IgG-specific cysteine protease, which cleaves IgG first into single-cleaved IgG (scIgG) with limited Fc-mediated functional capacity, then into F(ab')2 fragments. Imlifidase has been used to desensitize renal transplant recipients and is being investigated in sensitized HPST recipients and other antibody mediated diseases.